Index
- Biotoxin Monitoring Capabilities Expanded for Eastern Maine - Again!
- Monitoring Visible Algal Blooms in Western Maine
- The Nearshore Marine Resources Program: New Name, Expanded Capacity
- Another Successful Sampling Year: Thank You, Volunteers!
- Relaying Versus Depuration
Biotoxin Monitoring Capabilities Expanded for Eastern Maine - Again!
By Brianna King, Microbiologist II
Each year the DMR intensely monitors the entire Maine coast for blooms of phytoplankton species that are known producers of biotoxins detrimental to human health. These blooms impact valuable shellfish resources and when consumed, shellfish containing biotoxins can cause serious illnesses in the forms of Paralytic Shellfish Poisoning (PSP) and Amnesic Shellfish Poisoning (ASP). The DMR has been working for decades to build and improve upon not only our understanding of harmful phytoplankton bloom dynamics but also our response time to bloom events. In recent years new shellfish sampling techniques have been introduced, the latest laboratory analysis methods have been adopted, work has been done with partners to develop bloom forecasting techniques, and advanced laboratory equipment has been acquired for sample analysis.
In 2020, the Maine DMR Water Quality/Biotoxin Laboratory in Lamoine introduced a new piece of laboratory equipment, a High-Performance Liquid Chromatography (HPLC) instrument, for the in-house analysis of domoic acid, responsible for ASP. With this expansion in analysis capabilities, the Lamoine laboratory has been able to provide results from shellfish testing 1 -2 days earlier than previously when samples were brought to the DMR laboratory in Boothbay Harbor for analysis. By reducing sample transit time between labs, the duration of shellfish harvesting area closures in Eastern Maine due to domoic acid has also decreased. (For more details, please see the Winter 2021 newsletter.)
Now, thanks to federal funding, the DMR is on track to once again expand our analysis capacity, this time for the toxin responsible for PSP: saxitoxin. Similar to how ASP samples used to be analyzed, shellfish samples collected along the eastern half of the Maine coast for PSP analysis have historically been transported to the DMR Water Quality/Biotoxin laboratory in Boothbay Harbor for processing. These samples were then analyzed by our collaborators at Bigelow Laboratory.
This fall a second HPLC was purchased for the Lamoine laboratory’s in-house analysis of saxitoxin. Having the ability to test for the two most significant biotoxins within the Gulf of Maine - domoic acid (for ASP) and saxitoxin (for PSP) – at Lamoine is exciting progress in our endeavor to protect public health and will once again offer the potential to reduce biotoxin-related harvest area closures. With this new addition, we expect to be able to increase overall efficiency by reducing the additional demand on staff transporting samples to Boothbay Harbor and increasing the speed with which results are made available for PSP monitoring by an additional 1-2 days. We anticipate the analysis of shellfish samples for saxitoxin to begin this season at the Lamoine laboratory.
Monitoring Visible Algal Blooms in Western Maine
By Kirsten Johnston, Marine Resource Specialist II
Annually, DMR receives multiple accounts of visible marine algal blooms in Western Maine. Visible algal blooms are caused by the rapid growth of either toxin or non-toxin-producing phytoplankton. While nearly all visible blooms are non-toxic to humans who eat bivalve shellfish, these events are potential environmental hazards. This rapid growth of algae can deplete oxygen from the water column creating anoxic conditions. When large stretches of open water become low in oxygen (hypoxic), these areas become “dead zones” and can cause fish, shellfish, coral, and aquatic plant die-offs. Visible algal blooms also pose economic threats, as blooms can impact fisheries, aquaculture, and tourism. Most tracked blooms do not have marine mortalities associated with them and are gone within a matter of weeks.
In Western Maine, targeted sampling of visible, non-toxic algal blooms typically starts in mid-to-late July and lasts through the end of August as observations are provided to DMR or made by staff. These blooms can appear in many different colors but are commonly observed as reddish-brown or blue-green depending on the dominant plankton species in the bloom (Figures 1 and 2). In 2022, most of the large blooms in Western Maine were concentrated around the mid-coast region with sampling efforts focused between Freeport and Bristol (Figure 3).


Figures 1 and 2. Aerial images of Greenland Cove, Bremen (left), and Long Cove, Vinalhaven (right) taken on 08/16/2022. Photos were taken by Tyler Spillane
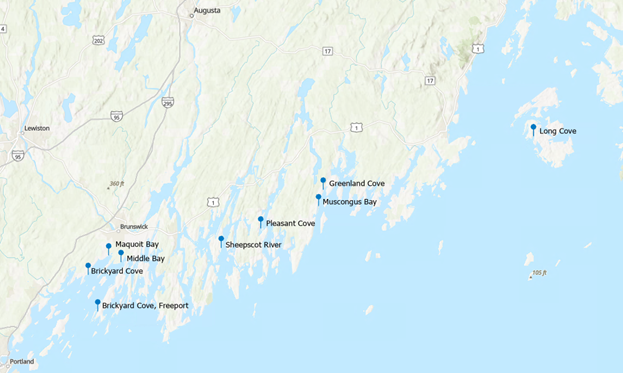
Figure 3. Whole water collection sites in 2022.
Non-toxic, visible algal blooms are sampled using whole water methods because these blooms are frequently dominated by fragile unarmored dinoflagellates. This protocol differs from filtered phytoplankton collection used to sample toxic blooms since the algae are in much higher densities and do not require concentrating the cells in a sample collection container and may be more fragile algae types than those associated with toxic blooms. To perform whole water sampling, a 2-liter container is flipped upside down and submerged completely below the surface of the water. The container is finessed until all air has escaped, and the lid is twisted into place while the container is still completely submerged. These samples are best stored at ambient air temperature, or in a cooler partially filled with seawater on very hot summer days. Unlike filtered phytoplankton samples, whole water samples are always read live, meaning no preservatives are added to the sample. This technique makes it much more difficult to identify and quantify plankton in the samples because the plankton can be zooming around under the microscope!
The Nearshore Marine Resources Program: New Name, Expanded Capacity
By Meredith White, Marine Resource Scientist IV
The Department’s Shellfish Program, which has assisted Maine municipalities with shellfish co-management for decades, has undergone a name change that came with expanded capacity. The change in name to the Nearshore Marine Resource Program more accurately reflects the responsibilities that already existed for the program in addition to municipal shellfish co-management, including state management of marine worms, periwinkles and whelks, mussels, subtidal resources, seaweed, and shellfish harvesting for depuration. The Maine administration and legislature approved the creation of a new senior scientist position to lead the program, as well as two new supporting scientists to expand the capacity of the program in addressing new and dynamic challenges confronting harvesters, which are caused by climate change. Meredith White was hired in November 2022 to supervise the program and Katie Tilton and Meryl Grady joined the program in January 2023. Rounding out the program are the existing Marine Resource Scientists (formerly Area Biologists) Hannah Annis, Ari Leach, and Heidi Leighton, who handle the majority of the Municipal Shellfish Program.
Moving into 2023, the Nearshore Marine Resource Program will be starting two new initiatives: a Municipal Shellfish Management Mini-Grant Program and a Long-Term Climate Change Sentinel Site Monitoring Program. The Mini-Grant Program will become an annual program through which municipalities can access funding to help them develop and test the efficacy of shellfish conservation measures. We are currently working on selecting 10 sites throughout Maine for the Long-Term Climate Change Sentinel Site Monitoring Program. For this, we are working with other research institutions and organizations throughout Maine to avoid duplication of effort, build monitoring capacity, and standardize methodology to build a climate change monitoring network beyond what any one program could support. We plan to conduct monthly comprehensive and quantitative surveys at each of the 10 sites, initially focusing on macroscopic species, but adding in microscopic species as the project continues. Observations, findings, and results from this monitoring will be shared with the public through annual reports.
Additionally, at the request of worm harvesters, we are planning a larval supply and recruitment study for sandworms and bloodworms in Westport Island and Wiscasset for the summer of 2023. This effort is intended to help understand the cause of a decline in observed worm abundance. We are developing a collaboration with academic researchers through the Maine eDNA Project to create molecular tools to detect worms, ideally reducing the labor needed for future studies.
Finally, we are excited to continue building communication with harvesters and stakeholders through the Regional Shellfish Meetings, which began in 2022, as well as through other efforts including additional stakeholder meetings, distribution of reports, and presentations. We look forward to presenting a poster about our Program’s projects at the 2023 Fishermen’s Forum on March 2 during the Shellfish Focus Day Poster Session/Social Hour at 3 pm. If you have any questions about the program, please reach out to meredith.m.white@maine.gov.
Another Successful Sampling Year: Thank You, Volunteers!
By Amy Webb, Microbiologist
We have had another successful sampling year thanks to the help of our dedicated water quality and phytoplankton volunteers. DMR’s phytoplankton monitoring program is designed to provide early warning of harmful algal blooms (HABs). Our biotoxin volunteers made up a significant portion of our phytoplankton monitoring efforts last year. Almost a quarter (22%) of the phytoplankton samples collected in 2022 were analyzed by volunteers across Maine’s coastline from Wells to Bar Harbor. By mid-December approximately 242 water samples had been collected, filtered, phytoplankton identified, counted, and reported to the DMR by trained volunteers. Just over 90% of the phytoplankton samples counted by volunteers contained at least one algae species known to produce toxins. For most types of phytoplankton, when routine water sampling detects cell concentrations (in terms of numbers of cells per liter of water) that are known to produce toxins, sampling of shellfish begins. If shellfish samples are shown to have toxins in concentrations higher than the limits specified by the National Shellfish Sanitation Program, areas are closed to shellfish harvesting.
Our water quality volunteers were no slouches last year either. Water quality volunteers collect water samples for analysis at our labs in Lamoine and Boothbay Harbor. These samples are used to monitor bacteriological water quality in growing areas. Water quality volunteers spanning Maines’ coastline from Freeport to Bar Harbor collected just over 1,320 water samples that were then transported per NSSP guidelines to Boothbay Harbor and Lamoine laboratory staff. Volunteers collect water samples at least 6 times per year at designated locations, the majority of which are accessible by land or boat. Over 60% of the water samples collected by our volunteers last year were collected by boat, a significant resource that is provided by the volunteer. The DMR would like to acknowledge and show appreciation for the time, effort, and resources our volunteers provide to the program. We look forward to working together with all our volunteers toward another successful sampling year in 2023.
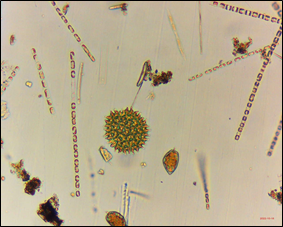
A photograph of a variety of phytoplankton species submitted by a volunteer from a sample collected at Fort Popham, Phippsburg on 10/17/22.
Relaying Versus Depuration
By James Becker, Seafood Technology Supervisor
Bivalve mollusks such as clams, oysters, and mussels filter feed seawater and gather planktonic food from their surrounding environment. If that environment is contaminated, these filter-feeding shellfish can accumulate toxins and pathogens and pose a health threat to human consumption. To lower bacteria to levels fit for humans to eat, effective methods have been developed and are used by some members of the shellfish industry. This allows the controlled harvest of bivalve shellfish from slightly polluted areas using a controlled process.
One process is called relaying, this is a method where contaminated shellfish are moved from a growing area in the Restricted or Conditionally Restricted classification to an Approved area (Figure 1). Shellfish are dispersed to settle on the bottom or placed in floating cages where they use the naturally occurring clean water to purge bacteria. Relaying can only be conducted through the use of a special permit provided by the Department of Marine Resources (DMR). The process is closely monitored, and testing is conducted to verify purging is successful.
Another process is depuration. This technique is conducted in land-based tank systems in sanitary seawater in a controlled environment (Figure 2). The shellfish accomplish the same end result, by purging and flushing bacteria by filter feeding in the clean seawater in which they are placed. Depuration can only be administered by a certified shellfish dealer and requires a monthly inspection of the system as well as lot testing.
Both processes, whether in a natural marine environment or in a land-based system, use the incredible purging ability of bivalve mollusks to reduce bacteria, with the goal of making them safe and approved by the authority for human consumption.

Figure 1. Floating shellfish cages for relay

Figure 2. Indoor tank system for depuration